Like most electronic components, the quality of countless subcomponents and processes directly affects the quality and performance of the finished product. With PCB-level connectors, those factors include pin material, type of plastic, the quality of the molded plastic bodies, coplanarity of the tails, the quality of the surface finish (plating), choosing the correct connector plating, the manufacturing/assembly process (putting the pins in the plastic), and packaging, among others.
Connector Plating Is Mission Critical
Plating affects the life and quality of the terminal or socket (i.e., wear); it impacts corrosion resistance, conductivity, solderability, and of course, cost.
Below is a list of FAQs, answered by Phil Eckert, Samtec’s Quality Engineering Manager, and Matt Brown, Principal Engineer, about all things pertaining to connector plating. BTW, the goal here is not to write a textbook about connector plating, chemistry, or metallurgy. We want to provide brief, to-the-point answers to the most commonly asked plating questions.
Why use gold plating?
Gold is generally specified for high reliability, low voltage, or low current applications. Gold is used in high-cycle applications because it’s rugged and has excellent wear properties (here’s an example of a high-cycle connector). Our gold is alloyed with cobalt, which increases the hardness. We also recommend gold for hostile environments, because it will remain free of oxides which could cause an increase in contact resistance.
Gold is a noble metal, which means it doesn’t react much to its environment.
Also, sometimes gold is a “matter of necessity” in that as connectors are miniaturized, contacts are too small to generate much normal force. So, low normal forces guide the need for Au plating.
The disadvantages of gold are primarily cost, then porosity at thinner plating levels, and some nuances regarding solderability. Specifically, many customers solder these “successfully,” but they are not soldering to the Au, because the Au dissolves in the molten solder. They are soldering to the nickel under the Au. So, technically, it is correct to say Au has poor solderability.
Why use tin plating?
Tin is a lower-cost alternative than gold, and has excellent solderability. Unlike gold, tin is not a noble metal. Tin plating starts to oxidize the moment it’s exposed to air. So a tin-plated contact system requires greater normal forces and a longer contact wipe area to break through this oxide film. Check out the brief video below.
Tin is often preferred due to its relatively low cost in applications with few mating cycles having the appropriate amount of normal force. (BTW, the connector in the video above is the SSW series)
What is the most popular connector plating option?
Selective gold-tin plating is Samtec’s most popular plating option because it provides designers with the best of both worlds. The contact area, the critical area where the contact interfaces the terminal pin and the signal is transferred, has the reliability of gold. The tail, which is soldered to the board, has a lower cost and improved solderability of tin.
Can gold be soldered?
This is kind of a loaded question. Some people say you should not solder gold-plated components to a PCB. Others say yes, but be cautious! You should be cautious because gold plating dissolves in solder, there’s the risk of solder bath contamination, and gold embrittlement.
Regarding embrittlement, once the gold weight contribution to the solder joint is 3% or 5%, embrittlement is a concern. Regarding gold dissolving in solder, and as explained above, solder paste is not soldering to the gold plate. The gold plate dissolves into the molten solder and the soldering takes place with the nickel underplate.
Can I mate gold and tin-plated connectors? Tell me about galvanic corrosion.
When choosing connectors for your application, it is important to choose mating connectors with similar plating materials in the contact areas. Dissimilar metals in the contact areas can result in galvanic corrosion. Galvanic corrosion typically occurs with metals of differing electronegativities.
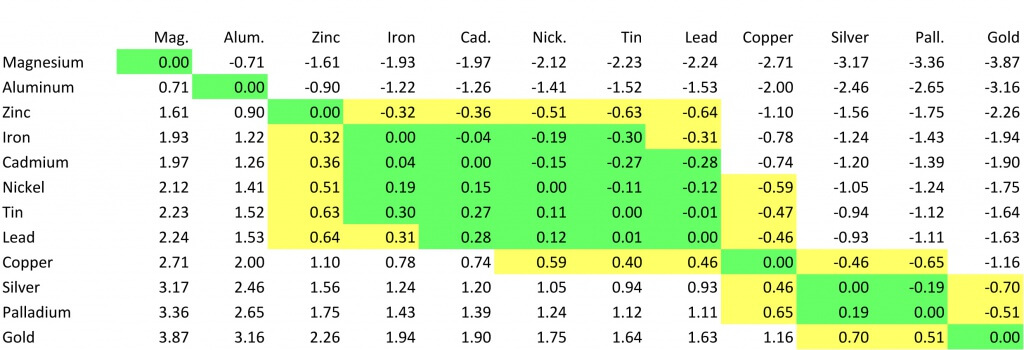
Below is a chart that shows how different plating materials react to one another with regard to their galvanic potential. According to this chart, the galvanic potential of gold and tin is high. If you’re interested in learning more, here’s the link to the blog that explains the chart.
Should the normal force of the contact dictate which plating to use?
The short answer is yes. With tin plating, the general guideline is 100 grams of normal force per mated contact to achieve a gas-tight connection. Oxides can potentially build to the point where there are electrically resistive layers on the surface finish of tin. To make a good connection you need enough normal force to break through that oxide. When I say these connectors require 100 grams of normal force, we’re not talking about microminiature connectors. You typically don’t see tin plating on these small connectors because it’s difficult to generate the appropriate amount of normal force.
With gold, because it’s a noble metal and does not react with contaminants and pollutants in the atmosphere, you can go with less normal force, like 30-40 grams.
You might be familiar with the phrase “30 – 40 grams, end of life,” which refers to the amount of contact normal force after accounting for thermal fatigue. This would be the contact normal force after testing is completed, and after it’s been exposed to higher temperatures. Often, the contact beams relax, but you still want 30 – 40 grams (with gold plating). Please note this is a general guideline; I’ve seen EOEMs with miniature connectors with less than 30 grams that function well.
We mentioned before that gold plating requires 30 – 40 grams of contact normal force. We’re giving a range because the normal force required can be affected by the contact design. Namely, the more points of contact the better.
What is the maximum continuous usage temperature of gold and tin plating?

With tin plating, it’s 105° C, and gold is 125° C. BTW, many customers use the terms “maximum continuous usage temperature” and “Operating Temperature Range” synonymously.
There’s also peak temperature, which refers to a temperature that the device is exposed to that is above the operating temperature for a limited time. Reactions occur when you’re above the operating temperature — for example, corrosion or oxidation on the surface — and all reactions are accelerated by heat. A general rule of thumb is every 10° C rise in temperature doubles the reaction rate. Therefore, once you get to a certain temperature, reactions that were happening slowly, that would not affect connector life for years, are suddenly happening very quickly, and can cause your connector to fail rapidly.
You should be aware of the connector’s Current Carrying Capacity (CCC). Running current through a connector increases the temperature in and around that connector, so the 105° C and 125° C is a consideration with that given current level.
What is the typical thickness of these connector plating options?
With tin plating, Samtec recommends at least 100 µ” of tin plating on the solder tail, and in many cases, greater than 100 µ” in the contact area. Some specifications don’t have a top end for tin plating. Because tin is inexpensive, we recommend 100 µ” minimum.
For gold, 30 µ” is the standard for reliability. We talk with many customers who require 30 µ” of gold in their designs, but we know many more who use 10 µ” of gold, or even down to 3 µ” of gold (called “gold flash”). OEMs may specify 30 µ” of gold if the connectors are required to pass Mixed Flowing Gas (MFG) tests, where the unmated contacts are exposed to chemicals like chlorine, hydrogen sulfide, nitrogen dioxide, and sulfur dioxide. Connectors with 30 µ” of gold will pass this test, but 10 µ” will usually fail due to porosity.
What are the maximum mating and unmating cycles of each connector plating?
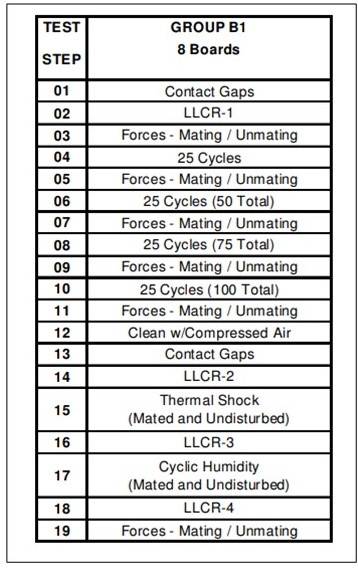
This is a frequently asked, difficult question. Many variables affect the number of cycles a connector system can handle. Of course, the first is the surface finish, and we know we’ll get more cycles out of gold. The base metal also plays a role, in that some base metals produce higher normal forces. A connector with very high normal forces can wear through the plating after many cycles.
We test our 30 µ” gold connectors to 100 cycles. There’s no doubt many customers are getting more than 100 cycles out of their connectors, but these testing standards tests are repeatable and reflect real-world applications. To the right, you see a typical Samtec test plan, and the tests performed after cycling.
Previously, we mentioned that the maximum continuous usage temperature for tin plating is 105° C, and it’s 125° C for gold. While we have connectors designed for power applications, most Samtec board-to-board components transfer signals, and the current level ranges from around 24 Amps on 5.00 mm pitch dual blade “power connector sets,” to 5 Amps – 6 Amps on a 2.54 mm pitch connector set, to around 2 Amps on 0.80 mm pitch microsystems.
The most simple, practical advice we can give is, if you require more cycles use gold, and the more cycles, the more gold. Cycle counts are also impacted by environmental conditions like temperature, moisture, humidity, and what pollutants and contaminants (e.g., sulfur and chlorine) are in the atmosphere.
What are the advantages and disadvantages of Sn/Pb plating?
Tin/lead plating has been on its way out the door for the last 30 years, and still is! By most measures, tin/lead plating produces a superior solder joint. Tin/lead also mitigates whisker growth, especially for military and space applications. Dendritic whisker growth can form on a pure tin plate, possibly creating a short. There are plenty of documented cases of whiskers on pure tin causing catastrophic failures.
While there are popularly documented cases of tin whiskers causing catastrophic failures, the consensus is that matte tin is normally accepted provided the plating is of good quality with some thought that a Ni underplate used with matte tin further decreases the chances for whiskers.
Tin/lead plating also has a lower eutectic temperature, which can help protect sensitive components as they are processed. Tin/lead plating allows oven temperatures to be set at 15° C to 30° C lower compared to a pure tin finish. For example, a lead-free oven might be set to 245° C to 260° C; leaded applications may be set to 230° C, and are frequently lower.
The disadvantages of tin/lead are obviously the environmental concerns as represented in protocols like RoHS and REACH. Some customers might experience availability issues with tin/lead-plated connectors, but that is uncommon.
What underplating is best? How thick should the underplating be?
Samtec underplates with 50 µ“ minimum of nickel. This increases durability, and it prevents base metal migration. A nickel underplating creates a physical barrier between the copper of the pin and the gold surface finish, or plating. Nickel is also inexpensive.
The downside to nickel is its ductility. Too thick of a layer can lead to cracking if the connector leads are formed or bent after plating (e.g., a right-angle forming process).
Do you recommend lubrication for connectors?
We rarely recommend lubrication for Samtec connectors, because it’s not necessary for most of our customers’ applications. Having said that, we’re not opposed to lubricating connectors, and you can use lubrication on our connectors.
For example, we have a standard lubrication option on a few of our connector series that have unusually high normal, mating, and unmating forces. In these situations, it’s possible the normal forces are so high that the connectors can be damaged during mating and unmating.
Also, lubrication adds a layer of protection against corrosion. We’ve had success using lubrication on both gold- and tin-plated contacts. Lubricants first came into wide use in tin-to-tin mating applications. Such mates can cause fretting corrosion, which is mitigated easily with lubrication. Gold on gold mates can use lubrication to seal pores and extend life in corrosive service environments.(1)
What are the voltage and amperage ratings for gold and tin connector plating?
The amount of current a contact system can carry is best indicated by current carrying capacity (CCC). The geometry of the contact system, the ambient conditions, the density and location of components on a customer’s PCB all play a role in the inevitable and natural rise in temperature as current passes through a connector. The goal is to hold the current to a level such that the maximum continuous use temperature is not exceeded. Therefore, the amount of current a connector system can tolerate is related to the temperature.
Does the base metal affect the functionality of the gold or tin plating?
We don’t recommend a surface finish based on the base metal of the connector pins. At Samtec, the base metal of most of our terminals and contacts is either Phosphor Bronze or Beryllium Copper, and both gold and tin work well with both of those base metals. Gold plating is common on Beryllium Copper contact systems, often because smaller, higher density, lower profile connector systems are usually made of Beryllium Copper. These smaller contact systems generate lower normal forces, in the 30 – 40 grams range, so Au-plated Beryllium copper is preferred.
Similarly, insertion and withdrawal forces are a consideration with tin-plated contact systems. As discussed in the first blog, tin-plated contact systems usually have a minimum thickness of 100 µ”, and are often 150 µ” or more, which can affect insertion and withdrawal forces, especially in high-density (i.e., high pin count) connector systems.
The plating combination of gold over nickel underplating was engineered for copper-based alloys.
What plating should I use for an edge card connector?

We recommend hard gold plating on the pins of an edge card connector; our gold is cobalt hardened. Likewise, we recommend the pads on the PCB, that mate with the connector also be cobalt-hardened gold. For the pads, we recommend a hardness of ASTM B 488 Type 2, Grade C. We recommend this because most edge card applications see a considerable number of mating cycles.
Are there new plating options or technologies on the horizon?
Let’s start with gold plating. Chemical manufacturers are trying to develop plating processes that reduce or eliminate the inherent porosity of plated gold. A goal is to get 10 µ” of Au to pass Mixed Flowing Gas testing.
Another potential avenue is lubricants. Lubricants continue to evolve and transfer into other technologies, including sealants. Base metal corrosion can be removed or greatly minimized if pores in the gold can be sealed.

Besides gold plating, a number of palladium options are also available. Most of these were researched because of the price of gold, but unfortunately, the price of palladium has spiked dramatically to the point where it is now not economical to use palladium instead of gold plating. Pure palladium, palladium-nickel, or palladium cobalt have been used as alternatives to hard gold; they typically receive a treatment of soft gold flash.
There’s also “diamond dust,” which refers to nanoparticles added to plating to refine their grain structures. There’s a lot of work underway on different types of particles.
Finally, some manufacturers are looking at changes to the nickel underplate, including alloys and “grain pinners.” These add a small amount of titanium to the nickel bath. This is a co-deposited alloy to “pin” the size of the nickel grains and improves its corrosion resistance.
What is the shelf life of gold- and tin-plated connectors?

The quick answer is Samtec recommends 12 months for Sn and 18 months for Au. The harder answer is, “it really depends.” Both of these surfaces are susceptible to oxidation in certain types of environments, and they both have to deal with the base metal migrating through the diffusion barrier (in our case, the nickel underplate), and into the connectivity layer (in our case, usually the gold).
First, connectors should be stored in a controlled environment – a stable room temperature and stable humidity. Second, they should exclude the typical industrial pollutants typically found in heavy manufacturing areas, like sulfur compounds and chlorine compounds. A connector can live long and prosper in such an environment.
As an aside, very occasionally we receive a question about contamination or corrosion on a connector. Typically, these customers are located close to the ocean, in an urban setting (where there are more likely to be pollutants in the atmosphere), and where the environment is warm/hot. The problem with what we just described is, that’s where most of the people in the world live – close to the ocean, in a warm environment, and in an urban setting.
What is that small, unplated line on the pin? Will it lead to corrosion?

That is a “bandoleer mark.” A bandoleer is a piece of stamped metal whose job is to carry the pin through the plating and manufacturing/assembly processes. Bandoleers are primarily used to hold square post pins used in terminal strip connector products (see this product, for example).
Bandoleer marks are always going to be present on the pins, and Samtec (as well as most other connector manufacturers) tries to locate these marks in a non-critical area of the pin. Real-world-speaking, this is rarely, if ever, an issue for our customers.
Does tin plating discolor in convection ovens? Is that a problem?
Yes, tin plating will discolor in convection ovens. It’s an inherent property that tin–plated pins begin to oxidize as soon as they are exposed to air. That’s one of the reasons why Samtec takes special precautions in storing connectors with tin-plated pins.
Oxygen will accelerate the oxidation process; every 10° C increase doubles the reaction rate. Therefore, sending a connector through a convection oven will increase the level of oxidation, even if it’s only for a short time. We do expect some discoloration in our tin-plated connectors as they are processed. Having said that, usually, we do not have quality issues.
Higher normal forces in the mating terminal pin/contact break through the oxidation layer, and flux is used in solder paste to reduce oxidation.
How can I prevent solder wicking?
Samtec tries to create an area on the pin that does not readily accept solder paste. This may be some type of nickel barrier that separates the tin from the gold plating. The solder will obviously wet to the tin but stop at the nickel. We are attempting to create a physical barrier between the tail (the part of the pin you want to solder) and the contact area (the part of the pin you don’t want to solder).
On many smaller connector systems, which don’t have much room to create a significant nickel barrier (i.e., the pins are very low profile (i.e., short)), some connector manufacturers will use laser ablation to burn away the gold, and in some cases, even the nickel or tin, to create a barrier layer to prevent wicking.
There’s also back stripping – which Samtec doesn’t offer – which strips away surface finish to expose a surface that is less likely to wet solder.
Does Samtec Use Silver or Nickel Plating?
Samtec does not offer silver plating. Silver is frequently used in very large connectors, where gold is cost prohibitive because of the amount of gold to cover the pin (we don’t want to put an ounce of gold on one connector). Silver is also used in applications that require extremely high temperatures, like high-power connectors. The disadvantage of silver is it reacts to sulfur in the air and forms a sulfate – which is ugly – and will inhibit contact. The good news is this oxidation is easily wiped away, so it’s really a cosmetic issue.
Samtec uses nickel as an underplate, but not as a surface finish. The nickel oxides that form are very resistive, so we don’t recommend them for signal applications. It’s extremely rare to see a nickel surface finish.
Should I Be Concerned About Fretting Corrosion?

Fretting corrosion is a legitimate concern, and it usually involves tin plating in high-vibration environments. Fretting corrosion is a micro-motion issue with movement typically less than .005″ (.127 mm). Just about any material that oxidizes is in danger of fretting corrosion under the right circumstances.
If a mated connector set is moving or sliding, even with minimal motion, a new layer of tin is exposed, which leads to oxidation. If that cycle continues, and the mated connector set is exposed to ongoing micro motions, the connector is continually trying to break through that oxide layer. About once every 12 to 18 months we receive a tin-plated connector set where the contact area has a grey, darkened circular/oval-shaped spot, which is a classic example of fretting corrosion.
The simplest answer to that problem is to switch to gold if possible, and if not, see if you can take steps to control the micro motions in the system?

Does The Lubricity of Matte Tin Plating Affect Crimping Discrete Wire?
Yes, it does. For environmental reasons, many connector companies have replaced Tin-Lead plating with bright acid tin or matte tin. Matte tin does not wear as well as bright acid tin (also called bright tin), so fragments of the matte tin can be deposited onto the wire crimping tools, requiring the tool to be cleaned frequently to prevent the build-up of tin in the tool. Our solution is we’ve kept the plating of our discrete wire connectors as bright tin. Bright tin is harder, slicker, and has much less build-up than matte tin.
What Surface Finish Should I Use For Temperatures Greater Than 125° C?
This is a difficult question because the industry hasn’t settled on an answer yet. There are a number of options available. As we discussed above, silver is well-suited for high temperatures. Palladium-nickel has proven to be a reasonably good alternative for gold, to get above 125° C. Designers who like to stick with gold are looking at new alloying elements. We previously discussed that we alloy our gold with cobalt for hardness. Other alloying elements include nickel or iron, both of which may have the ability to operate at higher temperatures, but test data is still out.
Is A Particular Underplate Recommended For Connector Pins That Are Exposed To Higher Temperature Solders?
Samtec still recommends a 50 µ” minimum nickel underplate in applications that use a higher-temperature solder. The issues with high temp solder typically relate to oxides forming on the surface of the gold or tin, and not migration of base metal through the nickel. Even at higher temperatures, the nickel diffusion layer is going to do its job. We’ve not had applications where customers run into issues where we’ve determined the root cause of the issue is the underplate.
Is It Important To Differentiate Between Bright Tin and Matte Finish Tin Plating?
Bright tin, compared to matte tin, is aesthetically pleasing and will provide a slightly lower coefficient of friction when mating with the other half of the connector. Bright tin will also slightly reduce the insertion force. Matte tin-plated connectors are better suited for the solder reflow process because they are less likely to discolor in elevated temperatures. Matte tin is “purer” in that it doesn’t have organic additives and will probably give you a better solder joint.
If you have questions, please direct them to [email protected].
(1) Samtec’s Kevin Meredith published a paper entitled “The Effects Of Lubrication On Connector Processing.” The objective of the test was to determine if any level of connector lubricant contamination in the lead region of the connector could result in processing defects. The effects of lubricant type, lead geometry, flux type, and processing technology were considered variables in this study.
In the paper Kevin details the test plans, and the processing results of several connector sets to meet IPC-A-610F standards; the connectors tested were the SEAM, ST4, HSEC8, and TFM series.
Of course, Kevin shares the conclusions of the tests. While I prefer to let the paper speak for itself, the essence of the conclusions is it is unlikely that the Santolube OS-138 used by Samtec will result in processing problems for any level of accidental lead contamination.